Abstract
Introduction: Granulocyte colony-stimulating factors (G-CSFs), when used to reduce the incidence, severity, and duration of febrile neutropenia (FN) in chemotherapy patients, may subsequently reduce direct medical costs associated with FN by reducing rates of hospitalization, serious infections, and the use of broad-spectrum antibiotics. While the cost per chemotherapy cycle for short-acting G-CSFs (ie, filgrastim [Neupogen®], filgrastim-sndz [Zarxio®], and tbo-filgrastim [Granix®]) depends on the frequency and number of doses administered and dose used (ie, 300 mcg or 480 mcg), the cost for the long-acting G-CSF pegfilgrastim (Neulasta® 6 mg) is relatively consistent, as it is dosed once per chemotherapy cycle and is effective for 14 days. Given this difference, the extent and duration required for short-acting G-CSF treatment determine the cost-efficiency of these agents relative to pegfilgrastim, when considering similar efficacy and safety across products. This cost-efficiency analysis evaluated the effective daily cost of G-CSFs per chemotherapy cycle, from a US payer perspective.
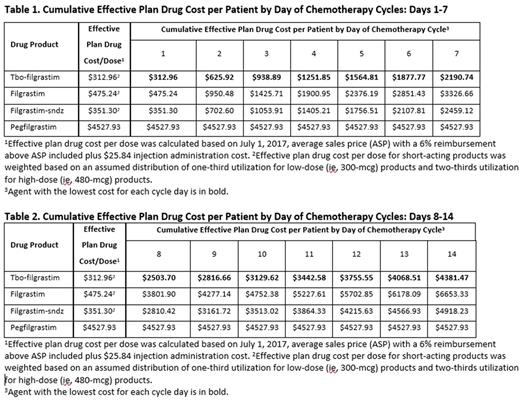
Methods: An interactive model was developed to simulate the relative cost for one chemotherapy cycle from 1 to 14 days for all currently marketed G-CSF products indicated to decrease the incidence or reduce the duration of FN in patients with non-myeloid malignancies treated with myelosuppressive chemotherapy. All agents were assumed to be provided at the health care provider (HCP) office, purchased through the patient's medical benefit, and administered by the HCP. An administration cost for each injection was included. Base-case data were derived from publicly available resources. Daily cost for short-acting G-CSFs was calculated using single dose drug acquisition cost and one administration fee per day, while daily cost for pegfilgrastim was calculated using the drug acquisition cost for one injection plus one administration cost. Additionally, the acquisition cost for short-acting agents was weighted according to the national utilization for low- (300 mcg) vs high-dose (480 mcg) products.
Results: The cost of tbo-filgrastim treatment ranged from $312.96 (1 dose) to $4381.47 (14 doses), the cost of filgrastim-sndz treatment ranged from $351.30 to $4918.23, and the cost of filgrastim treatment ranged from $475.24 to $6653.33, compared with $4527.93 for one injection of pegfilgrastim. Tbo-filgrastim is cost-efficient compared with pegfilgrastim through 14 doses. Pegfilgrastim becomes cost-efficient compared with filgrastim-sndz at 13 doses and with filgrastim at 10 doses.
Conclusion: Real-world data suggest that short-acting G-CSF products are used fewer than 14 days per chemotherapy cycle in clinical practice, while one dose of pegfilgrastim is efficacious for 14 days. Using acquisition costs plus administration costs for the injections, without considering the additional impact of health plan-contracted rates and manufacturer rebates for the drugs themselves, tbo-filgrastim is cost-efficient for a complete 14-day chemotherapy cycle compared with pegfilgrastim, while filgrastim-sndz and filgrastim are cost-efficient compared with pegfilgrastim through 12 doses and 9 doses, respectively. That is, short-acting products are more cost-efficient than pegfilgrastim in any chemotherapy cycle if fewer than 10 doses are needed. This finding assumes equal efficacy and safety among G-CSFs, and that all other chemotherapy-related patient characteristics are otherwise equal, including clinical appropriateness of prescribed duration of short-acting G-CSFs. G-CSFs reduce FN-associated direct medical costs and may differ in their ability to further reduce total direct medical costs of care for chemotherapy-treated patients when daily cost is considered in conjunction with necessary days of therapy.
James: Teva Pharmaceuticals: Consultancy. Trautman: Teva Pharmaceuticals: Consultancy. Szabo: Teva Pharmaceuticals: Employment. Tang: Teva Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal